What Laboratory Reports Will the Nurse Review Before Administering Albumin to a Patient?
- Review
- Open Access
- Published:
Management of hyperkalemia in the acutely ill patient
Annals of Intensive Care book nine, Commodity number:32 (2019) Cite this article
Abstract
Purpose
To review the mechanisms of activity, expected efficacy and side effects of strategies to control hyperkalemia in acutely ill patients.
Methods
We searched MEDLINE and EMBASE for relevant papers published in English language betwixt Jan 1, 1938, and July i, 2018, in accordance with the PRISMA Statement using the following terms: "hyperkalemia," "intensive intendance," "acute kidney injury," "acute kidney failure," "hyperkalemia handling," "renal replacement therapy," "dialysis," "sodium bicarbonate," "emergency," "acute." Reports from within the past 10 years were selected preferentially, together with highly relevant older publications.
Results
Hyperkalemia is a potentially life-threatening electrolyte abnormality and may cause cardiac electrophysiological disturbances in the acutely ill patient. Frequently used therapies for hyperkalemia may, withal, also be associated with morbidity. Therapeutics may include the simultaneous administration of insulin and glucose (associated with frequent dysglycemic complications), β-2 agonists (associated with potential cardiac ischemia and arrhythmias), hypertonic sodium bicarbonate infusion in the acidotic patient (representing a large hypertonic sodium load) and renal replacement therapy (effective but invasive). Potassium-lowering drugs tin can crusade rapid decrease in serum potassium level leading to cardiac hyperexcitability and rhythm disorders.
Conclusions
Handling of hyperkalemia should not just focus on the ability of specific therapies to lower serum potassium level but also on their potential side furnishings. Tailoring handling to the patient condition and state of affairs may limit the risks.
Background
Hyperkalemia is a potentially life-threatening electrolyte aberration [i,two,three]. Although there is no internationally agreed upon definition for hyperkalemia, the European Resuscitation Council defines hyperkalemia as a plasma level > 5.v mmol/L and severe hyperkalemia as > vi.5 mmol/L [4]. Hyperkalemia is associated with poor outcomes in many different settings, including the acutely ill patient [5, half-dozen]. In acute hyperkalemia, the main mortality risks are cardiac rhythm or conduction abnormalities [7, 8]. However, the actual causes of death in patients with hyperkalemia are poorly described, and the causal relationship between hyperkalemia and outcome remains controversial.
The aim of this review is commencement to describe mechanisms and the risk–benefit ratio of dissimilar strategies of hyperkalemia treatment and second, to propose a tailored treatment strategy. This will include a word of the effectiveness too every bit complications of renal replacement therapy, limiting the risk of hypoglycemia with judicious insulin and glucose administration, and the potential benefit and risks of hypertonic sodium bicarbonate.
Methods
We searched MEDLINE and EMBASE for relevant papers published in English between Jan 1, 1938, and July 1, 2018, in accordance with the PRISMA Statement using the following terms: "hyperkalemia," "intensive care," "acute kidney injury," "acute kidney failure," "hyperkalemia handling," "renal replacement therapy," "dialysis," "sodium bicarbonate," "emergency," "acute." Reports from within the past x years were selected preferentially together with highly relevant older publications.
Association between hyperkalemia and outcomes
The potassium ion (Grand+) is the nearly arable cation in the body. There is an estimated full reserve of 3000–4000 mmol in adults, of which but 60 mmol (2%) are extracellular [9]. Hyperkalemia is associated with poor outcomes in many dissimilar settings: in the general population [5, 6], in patients with cardiac and renal disease [x,11,12,thirteen] and in critically sick patients [fourteen]. In a retrospective study of hospitalized patients, Khanagavi et al. [five] constitute that astute kidney injury (AKI) and prolonged hyperkalemia are independent predictors of in-hospital mortality. In acute myocardial infarction, a serum potassium above 4.5 mmol/50 is associated with a college mortality [eleven]. More recently, Legrand et al. [15] identified that a serum potassium > 4.5 mmol/L in centre failure patients admitted to the emergency department (ED) is associated with an increased risk of death.
The net effect is a U-shaped mortality curve associated with potassium abnormalities [16,17,18,nineteen]. Several observational studies take identified hypokalemia as an independent risk cistron for poor effect [13, 16,17,18,nineteen]. This association raises concern regarding the potential for overcorrection, as may occur with some fast-acting potassium-lowering agents. Even so, these associations do not mean causality and thresholds for treating hyperkalemia remain debated.
Cardiac manifestations of hyperkalaemia
Although patients with hyperkalemia tin can present rarely with weakness progressing to flaccid paralysis, paresthesias, or depressed deep tendon reflexes, the clinical presentation of hyperkalemia is usually benign until cardiac rhythm or conduction disorders occur. Acme of extracellular potassium has several effects on myocardial electrophysiology that contribute to intracardiac conduction disturbances. The intracellular to extracellular potassium gradient lessens when extracellular potassium increases, thus decreasing the resting membrane potential. Elevated extracellular potassium as well increases membrane permeability for potassium, lowers membrane resistance, increases repolarizing currents, and shortens transmembrane activeness potential elapsing.
While rising serum potassium initially increases conduction velocity, it decreases it at higher levels [20]. Classic hyperkalemia electrocardiographic findings include signs of hyperexcitability such equally peaked T-waves (reflecting a decrease in the threshold for rapid depolarization). Further, altered conduction may manifest as PR prolongation, loss of P-waves, QRS widening, bradycardia, and ultimately a sine wave rhythm due to action potential shortening and prolongation of diastolic depolarization.
Chiefly, the correlation between potassium elevation and electrocardiographic (ECG) changes is poor. Severe hyperkalemia may manifest with minimal or atypical ECG findings [1,2,3, 21], including nonspecific ST segment modification or pseudo-Brugada syndrome (featuring wide QRS, elevation of the ST segment, J-point acme, T-wave inversion). On the contrary moderate hyperkalemia (< 6 mmol/L) may have life-threatening ECG findings. The electrocardiographic manifestations of hyperkalemia are largely influenced by rapid changes of plasma concentration [7], the gradient of potassium beyond the myocardial jail cell membrane, the effect of other ions (i.due east., sodium, calcium), besides as underlying cardiac illness [22]. Retrospective data plant a higher bloodshed rate in patients with hyperkalemia showing abnormal ECG findings [23]. Forth these lines, chronically dialyzed patients may prove no ECG signs of hyperkalemia despite high serum potassium levels. Altogether, more the absolute serum potassium level, therapeutic strategies should be guided by the cardiac consequences of hyperkalemia identified on the ECG (Fig. 1).
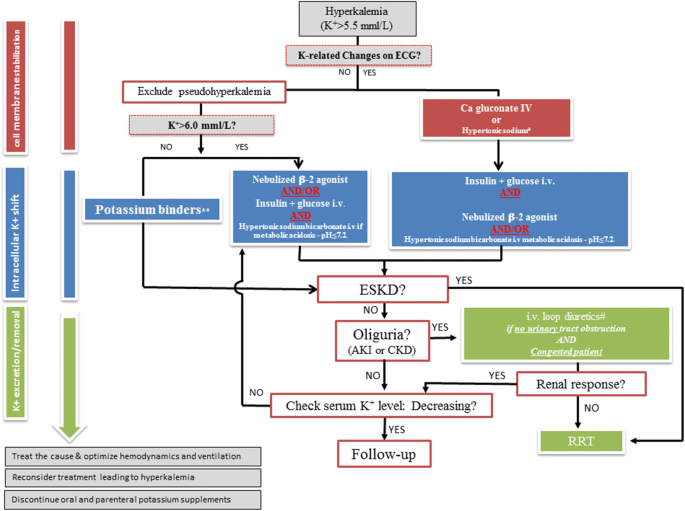
Suggested algorithm for hyperkalemia treatment in the acutely sick. *In case of Digitalis intoxication or hypercalcemia. **Sodium zirconium cyclosilicate and patiromer when available, kayexalate if not bachelor. ESKD end-phase kidney disease, AKI acute kidney injury, CKD chronic kidney illness, RRT renal replacement therapy
Causes of hyperkalemia in acutely ill patients
Factors associated with the development of hyperkalemia can exist classified into three categories, and include altered renal clearance of potassium (e.g., chronic kidney illness, acute kidney injury, renin–angiotensin–aldosterone organization inhibitor), release from the intracellular infinite (e.g., hemolysis, rhabdomyolysis, tissue injury) and contradistinct transfer to the intracellular infinite (e.g., acidosis, insulin arrears, β-adrenergic blockers, heparin) (Tabular array 1). Hyperkalemia in the patient with normal renal function is unusual and should prompt evaluation for pseudo-hyperkalemia if no ECG abnormalities consequent with hyperkalemia are identified (false superlative of potassium due to hemolysis occurring with blood depict and not reflective of the patient's plasma potassium concentration). While concomitant medications (eastward.g., potassium supplements, penicillin Chiliad, digoxin, nonsteroidal anti-inflammatory drugs, renin–angiotensin–aldosterone system inhibitor, amiloride, triamterene, trimethoprim, pentamidine) are often a contributor to hyperkalemia, in our experience they are rarely the only cause in acute settings.
Since the potassium pool is mostly intracellular, alteration of cellular potassium uptake can be a major contributors to hyperkalemia [24]. Hyperchloremic acidosis is frequent in acutely ill patients [25]. According to the Stewart'due south theory, the primary determinant of acid–base rest is the strong ion difference (SID), essentially determined by the deviation betwixt the strong cation (sodium) and the anions (chloride) [26]. A possible mechanism to explicate hyperkalemia related to hyperchloremic acidosis is that mineral acids (i.east., chloric) cannot freely diffuse into the intracellular compartment, they decrease extracellular pH. Low extracellular pH decreases the Na+–H+ exchange and inhibits the inward move of Na+. The subsequent fall in intracellular Na+ reduces Na+–K+-ATPase action, leading to a net subtract in K+ transfer into the cell and higher extracellular potassium levels. In this line, utilization of balanced solutions with physiological concentrations of chloride (i.east., Ringers lactate) prevents the development of mineral metabolic acidosis and is associated with lower serum potassium levels compared to NaCl 0.9% [25, 27, 28]. The effect of metabolic acidosis appears less prominent when organic acids accrue (i.e., lactate, phosphate). This is because organic acids can passively lengthened into the intracellular compartment, resulting in a larger fall in intracellular pH. The fall of intracellular pH stimulates inward Na+ movement and maintains Na+–Grand+-ATPase action, which minimizes the extracellular accumulation of potassium [29]. Ultimately, the increased intracellular Na+ concentration leads to the intracellular entry of potassium [29].
A special warning should be made with regards to the utilize of succinylcholine, classically used to induce paralysis in acutely sick patients for rapid sequence intubation. Succinylcholine induces skeletal muscle cell depolarization with an efflux of intracellular potassium by nicotinic receptor activation. In a population of critically ill patients, succinylcholine increased serum potassium on average 0.four mmol/L (interquartile range 0–0.7 mmol/Fifty) [xxx]. It should be avoided in patients with hyperkalemia and in patients with upwardly-regulation of nicotinic receptors, as they are at gamble of greater potassium peak. This includes those with anatomical denervation, prolonged administration of neuromuscular blocking drugs, burn injury, and prolonged immobilization [31]. Alternative to succinylcholine are bachelor in patients at risk of hyperkalemia (i.e., rocuronium).
Medical strategy
Starting time-line treatment in hyperkalemia with ECG abnormalities: myocardial protection
Calcium common salt
The intravenous administration of a calcium salt increases the cardiac threshold potential, the speed of impulse propagation and stabilizes the myocellular membrane, thus causing almost immediate normalization of the ECG abnormalities (Fig. ii). In 1950, Merrill et al. [32] found a beneficial consequence of intravenous calcium salt in 9 of ten patients with hyperkalemia. Four years later, this was confirmed by Chamberlain et al. [33], who reported five cases of an immediate upshot of intravenous calcium on ECG changes induced past astringent hyperkalemia (from 8.6 to 10 mmol/L). There are no randomized studies to bear witness its efficacy and its indications are based on expert opinion [34]. The consequence should exist immediate (within 5 min) when any hyperkalemia-related ECG changes are identified or suspected [33]. The protective event may last between xxx and 60 min [35]. Calcium administration in the case of hypercalcemia may be problematic. It also increased toxicity with digoxin overdose in animal models [34]. Nonetheless, this effect was found but at nonphysiologically high calcium concentrations [35]. The use of calcium in cases of hyperkalemia associated with digoxin toxicity was not associated with life-threatening dysrhythmias or mortality in human being studies [36,37,38]. Finally, calcium may cause tissue injury (i.e., skin necrosis) in case of extravasation [39]. The recommended dose is 10–20 mL of a 10% calcium salt (e.g., 1–2 g of gluconate or chloride).
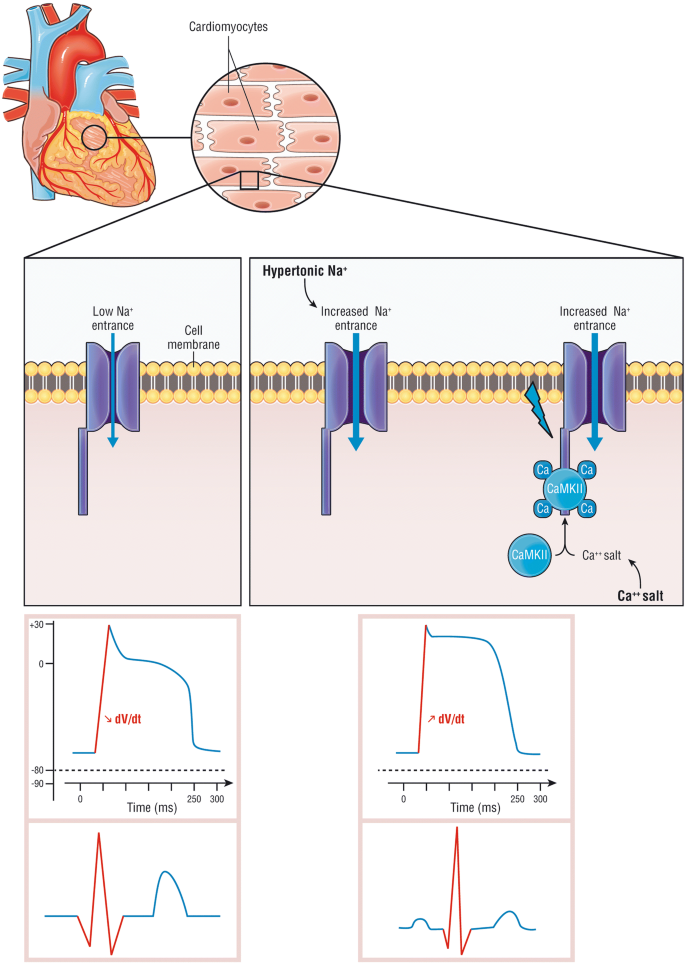
Cardiac issue of hypertonic sodium and calcium common salt during hyperkalemia. During hyperkalemia, resting membrane potential increases, derecruiting the sodium voltage gate channel Nav1.v (left panel). Calcium salts bind to calcium-dependent calmodulin and protein kinase II (CaMKII) and activates the sodium voltage gate channel leading to an intracellular sodium entrance (right panel). Calcium salt restores the channel activity though the calcium-dependent calmodulin (CaM), recruiting the voltage-gated aqueduct Nav1.5, increasing the intracellular sodium entrance, restore dV/dt phase 0 action potential and increment in the resting membrane potential. Hypertonic sodium increases extracellular sodium concentration and "forces" intracellular sodium archway (right panel). The bottom panel represents on the left the decrease of dV/dt phase 0 action potential due to hyperkalemia (Lesser left panel), restored by either calcium or hypertonic sodium (Bottom right panel)(Adapted from [forty, 41] with authorization)
Hypertonic sodium
Infusion of hypertonic sodium also increases the activity potential rise velocity in isolated cardiomyocytes [42]. In 1960, Greenstein et al. [43] studied the consequence of sodium lactate, sodium bicarbonate, and sodium chloride on ECG abnormalities induced by hyperkalemia in nephrectomized dogs. Infusion of hypertonic sodium increased the activity potential ascension velocity, which was depressed when isolated cardiomyocytes were exposed to increasing concentrations of potassium [42]. Taken together, these results suggest that hypertonic sodium acts as a membrane stabilizer and might be considered as an alternative to calcium in hyperkalemia-induced ECG changes when infusion of calcium is at risk. Furthermore, the fluid loading associated with hypertonic sodium bicarbonate may increase the glomerular filtration rate and renal potassium excretion in volume-depleted patients.
Intracellular potassium transfer
Hypertonic sodium bicarbonate
Although the information supporting the use of sodium bicarbonate as a treatment for hyperkalemia are controversial, it does have effects on serum potassium after infusion of hypertonic sodium bicarbonate. Some reported little effect on the potassium concentration in stable hemodialysis patients [44, 45]. In 1997, Ngugi et al. [46] observed that bicarbonate was less effective than salbutamol and insulin–dextrose in groups of 10 patients with end-phase renal disease (i.eastward., not acutely ill). Others reported effects on serum potassium. Schwarz et al. [47] reported that an infusion of 144–408 mmol of sodium bicarbonate over ii–4 h lowered the serum potassium by 2–three mmol/50 in 4 patients with severe acidosis.
In a recent randomized controlled trial (RCT), hypertonic sodium bicarbonate (4.two%) was administered to critically ill patients with severe metabolic acidaemia (pH < 7.two) [48]. There was no difference in the primary consequence (composite of expiry from any cause by 24-hour interval 28 or i organ failure at day 7), just the sodium bicarbonate group had significantly lower potassium concentrations compared to the control group and required renal replacement therapy less frequently. A more recent retrospective study also reported improved survival in septic patients with AKI stage 2 or iii and severe acidosis treated with sodium bicarbonates infusion [49]. Yet, the bear on on serum potassium was not reported.
Metabolic alkalosis, hypernatremia, hypocalcemia, and fluid overload are potential expected side effects of sodium bicarbonate (Table 2). Hypertonic sodium bicarbonate can cause hypocalcaemia in a pH dependent way and by straight calcium bounden [fifty]. In the Jaber et al. [48] study, more than patients in the bicarbonate grouping had ionized calcium lower than 0.9 mmol/L compared to patients in the placebo grouping (24% vs xv%, p = 0.0167) and 2 patients had a ionized calcium below 0.5 mmol/50 in the bicarbonates grouping versus none in the placebo group. Calcium is key for cardiac contractility. In an experimental model of lactic acidosis, Kimmoun et al. [51] reported improved myocardial elastance, aortic and mesenteric vasoreactivity when sodium bicarbonate was combined with calcium compared to sodium bicarbonate alone. Severe hypocalcemia can cause myocardial dysfunction and therefore ionized calcium should be monitored and ionized hypocalcemia corrected subsequently sodium bicarbonate infusion. Finally, even though sodium bicarbonate has been suspected of causing intracellular acidosis, this has not been confirmed in vivo [52]. We therefore recommend to use hypertonic sodium bicarbonate (eastward.g., 100–250 mL of 8.4% sodium bicarbonate over 20 min) in patients with metabolic acidosis (pH < 7.ii) or in patients with a contraindication to calcium administration (patients with hypercalcemia and/or severe digoxin intoxication), whether sodium bicarbonate is efficient in reducing serum potassium in patients without severe acidosis and the touch of the mechanism of metabolic acidosis need further exploration.
Insulin–dextrose
Insulin binds to the insulin receptor on skeletal muscle, activates the sodium–potassium adenosine triphosphatase, and leads to potassium transfer from the extracellular to intracellular space (Fig. 3) [53]. Although insulin–dextrose has never been tested versus placebo for the treatment of hyperkalemia, it shows like effects on serum potassium compared with salbutamol in a report of 20 patients [46, 54] simply with faster decrease in serum potassium with insulin (i.due east., 15 vs thirty min). Of note, combination of both farther lowered serum potassium compared to separate treatments. The major side effect of insulin is hypoglycemia, which has been reported to occur upwardly to 75% in subjects, depending of the protocol [55, 56]. One of the few blinded ED studies of hyperkalemia management plant a 17% charge per unit of clinically significant hypoglycemia afterward insulin–dextrose therapy [53].
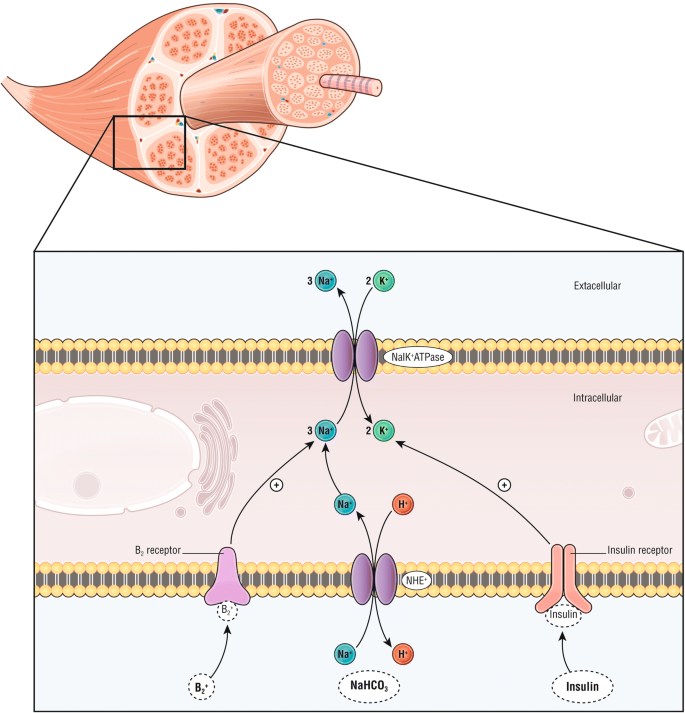
Activeness mechanisms of plasma lowering treatments by intracellular transfer. β-ii agonist (i.e., salbutamol) binds the β-2 receptor, insulin binds insulin receptors and sodium bicarbonate (NaHCOthree) induces an intracellular entrance of sodium through the Na+/H+ exchanger (NHE), all actuate the sodium–potassium adenosine triphosphatase (NaK+ ATPase) leading to a potassium transfer from the extracellular space to the intracellular space
Several studies suggest that a lower bolus dose of insulin may be safer. In two retrospective studies, similar potassium-lowering effects were plant with the assistants of either 5 or ten U of insulin (and 25 g of dextrose), but a higher incidence of hypoglycemia occurred with the college insulin dose [57, 58]. To limit hypoglycemia with the ten U insulin dose required using 50 thou to 60 g of dextrose [59]. Another strategy is to administer weight-based insulin dosing (0.1 U/kg of body weight upwardly to a maximum of ten U) to limit episodes of hypoglycemia without impacting potassium lowering [60]. Finally, using an infusion limited to xxx min led to a faster decrease in potassium, only less hypoglycemia as compared to continuous infusion [61]. Ultimately, because of the run a risk of hypoglycemia, claret glucose should be measured on an hourly basis for at to the lowest degree ii h, and potentially longer in the setting of renal failure [61]. While the risks of hypoglycemia have long been recognized, the risk of hyperglycemia is probably underappreciated. To summarize, using 5 U of insulin with 25 thousand of dextrose appears an effective and safe regimen. The touch on of exogenous administration of insulin and glucose on serum potassium and organ impairment in this setting is unknown. Intravenous administration of high doses of glucose to limit the risk of hypoglycemia may induce severe hyperglycemia, which has been associated with organ harm, vascular dysfunction and poor outcomes in different settings (i.e., centre failure, sepsis, critically ill patients) [62,63,64]. Critically ill patients frequently present with hyperglycemia and insulin resistance. We propose insulin–glucose as beginning-line treatment in patients with relative contraindication to β-two agonists (Tabular array 2) and patients with astringent hyperkalemia (i.e., ≥ 6.0 mmol/50 or associated with ECG changes).
β-ii agonists
Salbutamol (e.thousand., albuterol) is constructive at lowering potassium, without differences between nebulized or intravenous assistants, in terms of its efficacy [65, 66] fifty-fifty though effectiveness appears variable. However, salbutamol administered intravenously is associated with more than cardiovascular side effects than the nebulized route [67]. In one study of 10 patients treated with 10–20 mg salbutamol, the maximal decrease in potassium ranged from 0.4 to ane.22 mmol/50 [65, 66]. The peak event occurred between 60 and 90 min after administration, and the higher salbutamol dose was more efficient in lowering potassium. Due to systemic effects of salbutamol, regardless of the route of administration, side effects, such as tachycardia may also be of concern in patients with center failure or unstable angina. Finally, other consequences of β-2-agonists are hyperglycemia and increased plasma lactate. Impacts of treatments with β-blockers or efficacy in critically ill patients remain unexplored. Critically ill patients may present sympatho-adrenal activation (i.e., with tachycardia, vasoconstriction, hyperglycemia). We recommend the utilization of 10 mg nebulized salbutamol as starting time-line therapy in nonsevere hyperkalemia in spontaneous animate patients without tachycardia.
Increase potassium urinary excretion
Loop diuretics inhibit the NKCC2 channel at the apical surface of thick ascending limb cells along the loop of Henle. NKCC2 is a sodium–potassium–chloride cotransporter that reabsorbs (direct and indirectly) upwards to 25% of filtered sodium and chloride. Its blockade is responsible for most natriuretic effects of loop diuretics [68]. Loop diuretic assistants via the intravenous route is quickly followed past a similar dose dependent increase in both 24-h kaliuresis and natriuresis [69, 70]. The kaliuretic outcome is predominately a function of an increased tubular flow charge per unit and a college sodium concentration in the late nephron, both leading to an induction of the Na/One thousand+-ATPase that increases potassium excretion in the distal tubules and collecting duct [70]. Yet, ane major drawback of diuretics is the unpredictable natriuretic and kaliuretic effects, particularly in patients with AKI or heart failure. These patients may be resistant to the diuretic and kaliuretic effects of diuretics, thus making this a poor strategy to command astringent hyperkalemia. A "furosemide stress test" has been proposed in AKI patients to predict sustained AKI, with nonresponders defined as a urine output < 200 mL in the first 2 h afterward an infusion of one.0 or 1.5 mg/kg of furosemide [71]. In these nonresponders, alternative strategies to command hyperkalemia should not be delayed. Furthermore, loop diuretics should be titrated (0.ii–0.iv mg/kg in patient without AKI to 1–1.v mg/kg of furosemide in patients with AKI) and only considered in patients with fluid overload after excluding low intravascular volume and with close attention to the amount of diuresis to avoid boosted kidney insults resulting from iatrogenic hypovolemia. Finally, close monitoring for potential side effects, including the gamble of secondary hypovolemia and other electrolytes disturbances (i.e., dysnatremia, metabolic alkalosis, hypophosphatemia, hypomagnesaemia) is needed. To conclude, except in patients with symptomatic fluid overload, diuretics should not be considered as a therapy for hyperkalemia.
Gastro intestinal excretion
Sodium polystyrene sulfonate (SPS)
SPS exchanges sodium for calcium, ammonium, and magnesium in improver to potassium in the colon (Boosted file one: Figure S1) [72]. To date, no controlled trials in humans or animals have demonstrated that SPS increases fecal potassium losses, and no studies on the efficacy of SPS are available in the astute setting. Withal, serious gastrointestinal complications related to SPS, and attributed to sorbitol (co-administered with SPS to increase its delivery to the colon) have been described [73]. These include abdominal perforations, especially in patients with abnormal transit (e.g., patients in stupor or who are immediately postoperative). Furthermore, its use has been associated with edema and increases in blood pressure-likely related to the fact that it exchanges potassium for sodium. Due to its route of administration, its delayed and highly variable onset, and the potential for serious adverse side effects [35, 73], SPS is non a treatment of choice in the acutely ill patient.
Emerging treatment alternatives
Patiromer
Patiromer is a sodium-gratis, nonabsorbed, potassium-binding polymer, canonical in the USAUS and in the European union (EU) for management of hyperkalemia. In a recent meta-analysis of stage 2 and phase 3 trials, it was associated with a subtract in serum potassium of 0.21 ± 0.07 mmol/50 inside 7 h [74, 75]. Its long term efficacy and prophylactic was also shown in a 52-week trial [76]. Side furnishings include small-scale gastrointestinal intolerance and hypomagnesemia (7.i%) and edema due to substitution of potassium for sodium [75]. Patiromer has non been clinically tested in the emergency setting. Whether this chemical compound may enable the maintenance of normokalemia in emergency room patients is currently being tested (REDUCE study NCT: 02933450).
Sodium zirconium cyclosilicate (ZS-9)
ZS-9 is a crystal that is highly selective for potassium and ammonium ions exchanging sodium for potassium [77]. A recent meta-analysis of phase 2 and phase 3 studies ended that ZS-ix was effective in maintaining normokalemia with minor gastrointestinal side effects and edema [75]. Even though ZS-9 has not been specifically compared to existing alternatives for treatment of severe hyperkalemia in emergency conditions, Kosiborod et al. [78] recently described a subgroup of 45 patients with severe hyperkalemia (> 6 mmol/Fifty) who received a 10 grand dose of ZS-9. The median fourth dimension to a serum potassium level < half dozen.0 mmol/L was one.1 h, and the median time to a level ≤ v.five mmol/Fifty was iv.0 h, suggesting that this handling might exist considered in astringent acute hyperkalemia in patients with preserved gastrointestinal part. However, because of the lack of data in the acute setting and its potential delayed onset of action, it was not approved for acute hyperkalemia management in either the United states or in UE. An ongoing stage 2 study (NCT03337477) is evaluating the short term efficiency of ZS-9 plus insulin–dextrose versus insulin–dextrose alone in patients with acute hyperkalemia.
Renal replacement therapy
Indication of Renal replacement therapy
Astringent hyperkalaemia is a cardinal indication for renal replacement therapy (RRT) (due east.g., hemodialysis or hemofiltration) in acutely ill patients with AKI [eight]. However, what potassium concentration or other clinical indications (eastward.thou., significant ECG changes) should serve as triggers for RRT remain debated [8]. Even so, the literature does nonetheless provide some guidance [79]. In a contempo trial, a strategy of delayed RRT (with timing of RRT determined by serum creatinine or urine output) ultimately avoided RRT in many patients [80]. Not unexpectedly, medical treatment for hyperkalemia was more frequent in the delayed group, but the incidence of arrhythmias did not differ between groups. Of note, patients with potassium > 6, or > 5.5 mmol/50 despite medical treatment, were excluded, a gene limiting conclusions regarding acute therapy in those with the most astringent hyperkalemia. Another trial evaluated hypertonic sodium bicarbonate in critically sick patients with severe acidaemia (pH < 7.ii). They reported the bicarbonate grouping had a lower serum potassium, less need for RRT, and a longer delay to RRT in those patients ultimately requiring RRT [48]. Altogether these data suggest that medical handling of hyperkalemia (including hypertonic sodium bicarbonate in patients with metabolic acidosis) may be safe in critically ill patients with mild hyperkalemia. This medical handling could avoid or delay RRT onset in patients with AKI.
Renal replacement therapy and potassium dialysance
Renal replacement therapies (RRT) include diffusive (i.e., hemodialysis), convective (i.e., hemofiltration) and mixed modalities (east.thou., hemodiafiltration) in the acute setting. Potassium dialysance refers to the clearance of potassium in diverse RRT modalities. Body potassium dialysance and potassium flux depends on the gradient of potassium concentration between plasma and dialysate (or infusate using hemofiltration), claret and dialysate flow through the circuit, the modality (hemodialysis, hemofiltration, hemodiafiltration), and the dialyzer characteristics. Potassium mass transfer on the other side depends on handling fourth dimension and intracorporeal potassium kinetics (Fig. 4). Since potassium freely and totally diffuses throughout the dialyzer membrane, it is speedily and effectively removed during hemodialysis. In the setting of high blood and dialysate flow and depression dialysate potassium concentration, serum potassium drops within minutes of initiation. Since intracorporeal potassium kinetics carry as a multi-compartmental model, serum potassium volition decrease more slowly after two h of hemodialysis and rebound after stopping the therapy. Of note, hyperosmolarity, or treatments shifting potassium from the extracellular to the intracellular space before the dialysis session (i.e., β-2 agonists, sodium bicarbonate, insulin, glucose), volition subtract potassium dialysance.
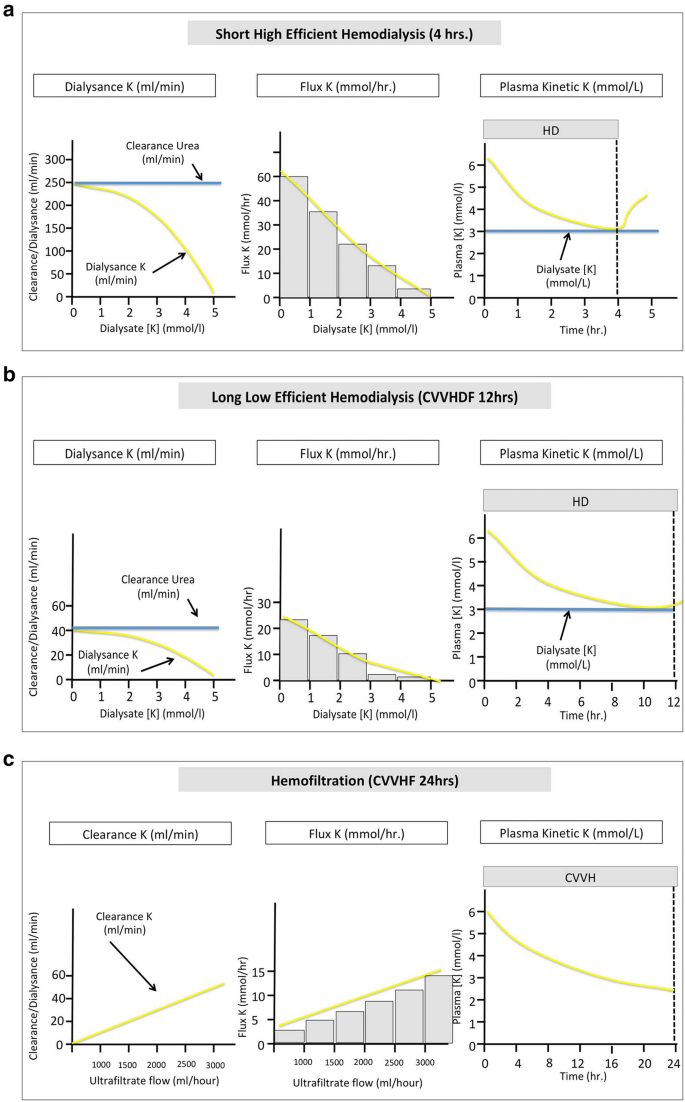
Action mechanisms of hypokalemic treatments by intracellular transfer. a Potassium dialysance, flux and plasma kinetic under short loftier efficient hemodilaysis. b Potassium dialysance, flux and plasma kinetic under long low efficient hemodilaysis. c Potassium clearance, flux and plasma kinetic nether hemofiltration. K potassium, CVVHD continuous venovenous hemodialysis, CVVHF continuous venovenous hemofiltration
Continuous RRT, including hemofiltration (i.east., convective technique), is the nearly frequently used modality in the intensive care unit of measurement. Using convective techniques, flux of potassium through the membrane depends on the ultrafiltration rate and the serum potassium level (Fig. iv). When combined techniques are used (i.e., hemodiafiltration), elimination of potassium depends mostly on the diffusive transfer through the membrane. Continuous depression flow techniques have a slower decrease in serum potassium concentrations, and the serum potassium will tend to approach dialysate (with diffuse techniques) or infusate concentration (with convective techniques) inside few hours after initiation without rebound. Hemofiltration using mild to high cut-off membranes as well allows college myoglobin removal in patients with rhabdomyolysis.
RRT volition naturally be a second line strategy. In our view, the option of RRT modality will largely depend on the available techniques. The efficacy and tolerance will nevertheless largely rely on RRT prescription. Using brusque high efficiency dialysis (intermittent dialysis) will require high claret and dialysate menstruum to remove sufficient amount of potassium (e.g., blood flow 250 mL/min and dialysate menses 500 mL/min) allowing rapid driblet of serum potassium simply with a adventure a rebound later on stopping RRT (Fig. 4). Clearance of potassium using continuous hemofiltration is proportional to ultrafiltrate rate (Fig. four). We therefore advise a high ultrafiltration charge per unit at the initiation of the technique (e.yard., ≥ 45 mL/kg/h) when using this modality. This ultrafiltration charge per unit can exist lowered when serum potassium is controlled (east.one thousand., 25 mL/kg/h).
Both techniques expose the patient to the risk of secondary hypokalemia. Importantly, both hyperkalemia and a rapid subtract in serum potassium are associated with cardiac events and sudden expiry in patients with stop-stage kidney disease [81, 82]. Long inter-dialytic periods betrayal patients to consequences of hyperkalemia and cardiac conduction disorders while intradialytic periods and postdialytic periods are associated to increase cardiac excitability and arrhythmic disorders. Rapid decreases in serum potassium using a potassium dialysate concentration ≤ 2 mmol/L was associated with a doubling of risk of sudden cardiac abort in a recent study [82]. This arrhythmogenic propensity of RRT is enhanced by simultaneous combined stresses including ischemia (hypovolemia), hypoxia, electrolyte changes (calcium, magnesium, citrate, acetate) and potential removal of cardiac medications. Studies have shown that the frequency of premature ventricular contractions during dialysis is less common when using a dialysate potassium concentration of ii.0–iii.0 mmol/50, compared ≤ 2.0 mmol/L [83]. More than recently, Ferrey et al. [84] examined the association of dialysate potassium concentration with all-cause mortality chance in chronic hemodialysis patients. They observed that a dialysate potassium concentration of 1 mEq/Fifty was associated with college mortality compared to higher concentrations. Taken altogether, these data advise using a potassium dialysate concentration ≥ ii.0 mmol/L to avert a too rapid drop in serum potassium using dialysis. Treatment of hyperkalemia using peritoneal dialysis has been described anecdotally and appears feasible when alternatives are not readily available [85]. Alternatives to prevent rapid and profound drop of serum potassium is to use low flow techniques (i.east., continuous hemofiltration, continuous hemodialysis or slow low efficiency or extended dialysis) (Fig. 4) once astute astringent hyperkalemia has been controlled. Continuous techniques will farther largely prevent rebound of serum potassium observed afterwards intermittent dialysis. Finally, extended or continuous session with high flow should be considered in patients with ongoing uncontrolled cause of hyperkalemia (i.e., rhabdomyolysis, tumor lysis syndrome).
Who should be treated for hyperkalemia?
Even though hyperkalemia has been associated with mortality in different settings [v], the potential side effects of hyperkalemia treatment should not exist overlooked. Tailoring treatment to the patient status and situation might limit the risk of nether or over-treating hyperkalemia [34].
The evaluation of hyperkalemia should e'er include assessment for the rapid demand of membrane stabilization treatment (i.eastward., calcium or hypertonic sodium solutions) and should be considered in patients with cardiac conduction or rhythm abnormalities (Figs. 1 and 5). When the clinical scenario and absence of ECG changes practice not support the likelihood of hyperkalemia, the potassium measurement should be repeated to exclude factitious hyperkalemia (or pseudo-hyperkalemia). A result of kalemia in delocalized biochemistry (i.e., claret gas analyzer) could probably be used to detect hyperkalaemia and start a treatment in high-gamble patients (e.g., patients with severe metabolic acidosis, AKI or CKD).
Efficacy and tolerance of treatment may vary widely according to the clinical scenario (Table 2). Insulin–glucose infusion appears to be appropriate for severe hyperkalemia due to its efficacy and reproducible lowering of serum potassium levels, with close serum glucose monitoring (Fig. 5). However, the impact of this regimen in critically ill patients with insulin resistance or dysglycemia remains unclear. Hypertonic sodium bicarbonate combines fluid loading, cardiac membrane stabilization and serum potassium lowering and is virtually advisable in patients with severe metabolic acidosis, AKI and hypovolemia. Aerosolized β-two agonists are more easily used in spontaneously animate patients and appear to take similar efficacy to the insulin–dextrose combination in lowering serum potassium. Nonetheless, the utilize of β-two agonists in patients with cardiac hyperexcitability, baseline high sympathetic activeness or with unstable coronary disease is potentially associated with severe side effects or decreased efficacy. In add-on, efficacy in mechanically ventilated patients is unknown. Serial serum potassium measurements after outset-line treatment let providers to assess the initial response and demand for a second line strategy. RRT is normally required in patients with severe AKI with oliguria or anuria who are not expected to rapidly recover (e.yard., AKI unresponsive to hemodynamic optimization, unresponsive to diuretics), in patients with end-stage chronic kidney disease admitted for an acute condition and in the setting of astringent AKI and hyperkalemia (i.eastward., > 6.5 mmol/L) and in patients with hyperkalemia resistant to medical therapy [eight, 34].
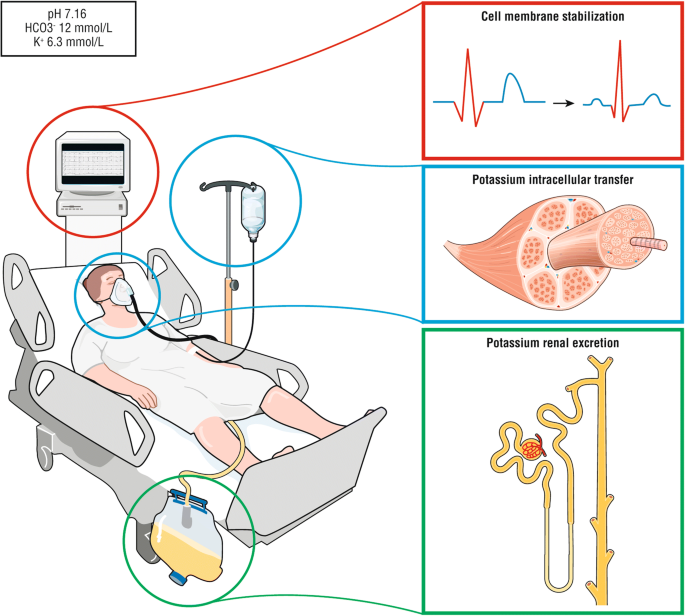
First-line treatment of hyperkalemia. During hyperkalemia with ECG modifications, first-line therapy should consist on cardiomyocyte stabilization using calcium salt or hypertonic sodium (red panel), second line therapy on treatment leading to a fast transfer of potassium from extracellular to intracellular space using either insulin–glucose i.v, aerosol of β2 agonist and/or sodium bicarbonate (in case of metabolic acidosis and hypovolemic patient) depending of the patient's comorbidities and clinical status. Insulin–glucose is recommended equally the first-line treatment in severe hyperkalemia (i.e., above half dozen.5 mmol/L) simply close glucose monitoring is mandatory. β2 agonists can be used in spontaneously breathing patients but with safety concerns in patients with unstable angina or cardiac failure. Hypertonic sodium bicarbonate should probably be restricted to hypovolemic patients with metabolic acidosis (blue panel). Strategies increasing potassium renal excretion decreases the total potassium pool (i.e., hemodynamic optimization and correction of acute kidney injury or loop Henle diuretics in patients with fluid overload) (light-green panel). Indications of renal replacement therapy are patients with severe acute kidney injury associated to severe hyperkalemia or persistent hyperkalemia despite first-line medical handling
Finally, identification and treatment of the cause and contributing factors of hyperkalemia should be performed simultaneously. Identification of the cause of AKI and rapid correction of contributing factors of AKI may allow faster recovery.
Decision
Recognition of hyperkalemia-related ECG changes is central in the choice of strategy to treat the patient. ECG changes should prompt urgent medical intervention including both cardiac protection and potassium-lowering handling. Tailoring treatment of hyperkalemia to the patient condition and situation volition limit the risks of treatments side effects. Efficacy and tolerance remain even so poorly explored in acute setting. There is a need for further inquiry to evaluate both efficacy and side effects of different strategies in the acute setting.
Abbreviations
- K+ :
-
potassium ion
- AKI:
-
acute kidney injury
- ED:
-
emergency department
- ECG:
-
electrocardiographic
- SID:
-
strong ion difference
- RCT:
-
randomized controlled trial
- NKCC:
-
Na–K–Cl cotransporter
- SPS:
-
sodium polystyrene sulfonate
- US:
-
USA
- EU:
-
European Union
- ZS-nine:
-
sodium zirconium cyclosilicate
- RRT:
-
renal replacement therapy
References
-
Mattu A, Brady WJ, Robinson DA. Electrocardiographic manifestations of hyperkalemia. Am J Emerg Med. 2000;18:721–9.
-
Freeman K, Feldman JA, Mitchell P, Donovan J, Dyer KS, Eliseo L, et al. Effects of presentation and electrocardiogram on time to treatment of hyperkalemia. Acad Emerg Med. 2008;xv:239–49.
-
Montague BT, Ouellette JR, Buller GK. Retrospective review of the frequency of ECG changes in hyperkalemia. Clin J Am Soc Nephrol. 2008;3:324–xxx.
-
Truhlář A, Deakin CD, Soar J, Khalifa GEA, Alfonzo A, Bierens JJ, et al. European resuscitation council guidelines for resuscitation 2015. Resuscitation. 2015;95:148–201.
-
Khanagavi J, Gupta T, Aronow WS, Shah T, Garg J, Ahn C, et al. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;2:251–7.
-
Phillips BM, Milner S, Zouwail S, Roberts G, Cowan M, Riley SG, et al. Severe hyperkalaemia: demographics and outcome. Clin Kidney J. 2014;seven:127–33.
-
Winkler AW, Hoff HE, Smith PK. Electrocardiographic changes and concentration of potassium in serum following intravenous injection of potassium chloride. Am J Physiol Leg Content. 1938;124:478–83.
-
Kellum JA, Lameire Due north, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, et al. Work group membership. Kidney Int. 2012;2:1.
-
Lee Hamm L, Hering-Smith KS, Nakhoul NL. Acid-base and potassium homeostasis. Semin Nephrol. 2013;33:257–64.
-
Jain N, Kotla South, Little BB, Weideman RA, Brilakis ES, Reilly RF, et al. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol. 2012;109:1510–3.
-
Goyal A, Spertus JA, Gosch K, Venkitachalam Fifty, Jones PG, Van den Berghe G, et al. Serum potassium levels and mortality in acute myocardial infarction. JAMA. 2012;307:157.
-
Brunelli SM, Du Mond C, Oestreicher N, Rakov V, Spiegel DM. Serum potassium and short-term clinical outcomes among hemodialysis patients: impact of the long interdialytic interval. Am J Kidney Dis. 2017;lxx:21–9.
-
Collins AJ, Pitt B, Reaven North, Funk S, McGaughey Thousand, Wilson D, et al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017;46:213–21.
-
McMahon GM, Mendu ML, Gibbons FK, Christopher KB. Association betwixt hyperkalemia at critical intendance initiation and mortality. Intensive Care Med. 2012;38:1834–42.
-
Legrand Grand, Ludes P-O, Massy Z, Rossignol P, Parenica J, Park J-J, et al. Association betwixt hypo- and hyperkalemia and issue in acute center failure patients: the role of medications. Clin Res Cardiol Off J Ger Carte Soc. 2018;107:214–21.
-
Hoss S, Elizur Y, Luria D, Keren A, Lotan C, Gotsman I. Serum potassium levels and outcome in patients with chronic heart failure. Am J Cardiol. 2016;118:1868–74.
-
Ahmed A, Zannad F, Love TE, Tallaj J, Gheorghiade M, Ekundayo OJ, et al. A propensity-matched study of the clan of low serum potassium levels and mortality in chronic heart failure. Eur Heart J. 2007;28:1334–43.
-
Luo J, Brunelli SM, Jensen DE, Yang A. Clan betwixt serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol. 2016;11:xc–100.
-
Rossignol P, Girerd North, Bakris G, Vardeny O, Claggett B, McMurray JJV, et al. Impact of eplerenone on cardiovascular outcomes in heart failure patients with hypokalaemia. Eur J Eye Fail. 2017;nineteen:792–9.
-
Lyons CJ, Burgess MJ, Abildskov JA. Furnishings of acute hyperkalemia on cardiac excitability. Am Center J. 1977;94:755–63.
-
Aslam Due south, Friedman EA, Ifudu O. Electrocardiography is unreliable in detecting potentially lethal hyperkalaemia in haemodialysis patients. Nephrol Dial Transplant. 2002;17:1639–42.
-
Burchell HB. Electrocardiographic changes related to disturbances in potassium metabolism. J Lancet. 1953;73:235–eight.
-
Durfey N, Lehnhof B, Bergeson A, Durfey S, Leytin Five, McAteer One thousand, et al. Severe hyperkalemia: Tin the electrocardiogram chance stratify for short-term adverse events? W J Emerg Med. 2017;xviii:963–71.
-
Kovesdy CP, Appel LJ, Grams ME, Gutekunst Fifty, McCullough PA, Palmer BF, et al. Potassium homeostasis in health and affliction: a scientific workshop cosponsored by the National Kidney Foundation and the American Lodge of Hypertension. J Am Soc Hypertens. 2017;11:783–800.
-
Yunos NM, Kim IB, Bellomo R, Bailey Grand, Ho L, Story D, et al. The biochemical effects of restricting chloride-rich fluids in intensive care. Crit Intendance Med. 2011;39:2419–24.
-
Stewart PA. Independent and dependent variables of acid-base control. Respir Physiol. 1978;33:9–26.
-
O'Malley CMN, Frumento RJ, Hardy MA, Benvenisty AI, Brentjens TE, Mercer JS, et al. A randomized, double-blind comparison of lactated Ringer's solution and 0.9% NaCl during renal transplantation. Anesth Analg. 2005;100:1518–24.
-
Khajavi MR, Etezadi F, Moharari RS, Imani F, Meysamie AP, Khashayar P, et al. Effects of normal saline vs. lactated Ringer's during renal transplantation. Ren Fail. 2008;30:535–9.
-
Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol. 2015;x:1050–60.
-
Blanié A, Ract C, Leblanc P-Eastward, Cheisson 1000, Huet O, Laplace C, et al. The limits of succinylcholine for critically ill patients. Anesth Analg. 2012;115:873–9.
-
Strayer RJ. Succinylcholine, rocuronium, and hyperkalemia. Am J Emerg Med. 2016;34:1705–6.
-
Merrill JP, Levine HD, Somerville W, Smith S. Clinical recognition and treatment of astute potassium intoxication. Ann Intern Med. 1950;33:797.
-
Chamberlain MJ. Emergency treatment of hyperkalaemia. Lancet Lond Engl. 1964;ane:464–7.
-
Rossignol P, Legrand 1000, Kosiborod M, Hollenberg SM, Peacock WF, Emmett M, et al. Emergency management of severe hyperkalemia: guideline for best practice and opportunities for the future. Pharmacol Res. 2016;113:585–91.
-
Alfonzo AVM, Isles C, Geddes C, Deighan C. Potassium disorders—clinical spectrum and emergency management. Resuscitation. 2006;lxx:10–25.
-
Smith PK. Calcium and digitalis synergism: the toxicity of calcium salts injected intravenously into digitalized animals. Curvation Intern Med. 1939;64:322.
-
Nola GT, Pope S, Harrison DC. Cess of the synergistic human relationship betwixt serum calcium and digitalis. Am Heart J. 1970;79:499–507.
-
Levine M, Nikkanen H, Pallin DJ. The furnishings of intravenous calcium in patients with digoxin toxicity. J Emerg Med. 2011;40:41–half-dozen.
-
Pacheco Compaña FJ, Midón Míguez J, de Toro Santos FJ. Lesions associated with calcium gluconate extravasation: presentation of 5 clinical cases and analysis of cases published. Ann Plast Surg. 2017;79:444–9.
-
Robert T, Joseph A, Mesnard Fifty. Calcium common salt during hyperkalemia. Kidney int. 2016;90:451–2.
-
Robert T, Burbach Yard, Joseph A, Mesnard 50. Sodium is the secret re-agent of bicarbonate therapy during hyperkalemia. Kidney Int. 2016;xc:450–one.
-
Ballantyne F, Davis LD, Reynolds EW. Cellular ground for reversal of hyperkalemic electrocardiographic changes past sodium. Am J Physiol. 1975;229:935–40.
-
Greenstein Southward, Goldburgh WP, Guzman SV, Bellet S. A comparative analysis of molar sodium lactate and other agents in the treatment of induced hyperkalemia in nephrectomized dogs. Circ Res. 1960;8:223–33.
-
Gutierrez R, Schlessinger F, Oster JR, Rietberg B, Perez Go. Effect of hypertonic versus isotonic sodium bicarbonate on plasma potassium concentration in patients with terminate-stage renal disease. Miner Electrolyte Metab. 1991;17:297–302.
-
Allon M, Shanklin Northward. Effect of bicarbonate administration on plasma potassium in dialysis patients: interactions with insulin and albuterol. Am J Kidney Dis Off J Natl Kidney Found. 1996;28:508–14.
-
Ngugi NN, McLigeyo SO, Kayima JK. Handling of hyperkalaemia by altering the transcellular slope in patients with renal failure: outcome of various therapeutic approaches. Due east Afr Med J. 1997;74:503–ix.
-
Schwarz KC, Cohen BD, Lubash GD, Rubin AL. Severe acidosis and hyperpotassemia treated with sodium bicarbonate infusion. Apportionment. 1959;19:215–20.
-
Jaber S, Paugam C, Futier E, Lefrant J-Y, Lasocki South, Lescot T, et al. Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the intensive intendance unit (BICAR-ICU): a multicentre, open-label, randomised controlled, phase 3 trial. Lancet. 2018;392:31–xl.
-
Zhang Z, Zhu C, Mo L, Hong Y. Effectiveness of sodium bicarbonate infusion on mortality in septic patients with metabolic acidosis. Intensive Care Med. 2018. https://doi.org/10.1007/s00134-018-5379-2.
-
Cooper DJ, Walley KR, Wiggs BR, Russell JA. Bicarbonate does not improve hemodynamics in critically ill patients who have lactic acidosis. A prospective, controlled clinical study. Ann Intern Med. 1990;112:492–8.
-
Kimmoun A, Ducrocq N, Sennoun N, Issa K, Strub C, Escanyé J-M, et al. Efficient extra- and intracellular alkalinization improves cardiovascular functions in astringent lactic acidosis induced past hemorrhagic daze. Anesthesiology. 2014;120:926–34.
-
Levraut J, Labib Y, Chave South, Payan P, Raucoules-Aime M, Grimaud D. Upshot of sodium bicarbonate on intracellular pH under unlike buffering weather. Kidney Int. 1996;49:1262–vii.
-
Ho K. A critically swift response: insulin-stimulated potassium and glucose transport in skeletal muscle. Clin J Am Soc Nephrol. 2011;half dozen:1513–half dozen.
-
Allon One thousand, Copkney C. Albuterol and insulin for treatment of hyperkalemia in hemodialysis patients. Kidney Int. 1990;38:869–72.
-
Rafique Z, Kosiborod Thou, Clark CL, Vocalizer AJ, Turner S, Miller J, et al. Study design of Real World Show for Treatment of Hyperkalemia in the Emergency Department (REVEAL-ED): a multicenter, prospective, observational study. Clin Exp Emerg Med. 2017;4:154–nine.
-
Peacock WF, Miller J, Char D, Clark CL, Singer AJ, Rafique Z, et al. 1* Burden of hyperkalemia and treatment patterns in the Emergency Department setting: results from the REVEAL-ED Study. Ann Emerg Med. 2016;68:S1.
-
LaRue H, Peksa GD, Shah South. A comparison of insulin doses for the treatment of hyperkalemia in patients with renal insufficiency. Pharmacother J Hum Pharmacol Drug Ther. 2017. https://doi.org/10.1002/phar.2038.
-
McNicholas BA, Pham MH, Carli K, Chen CH, Colobong-Smith Northward, Anderson AE, et al. Handling of hyperkalemia with a low-dose insulin protocol is effective and results in reduced hypoglycemia. Kidney Int Rep. 2018;three:328–36.
-
Coca A, Valencia AL, Bustamante J, Mendiluce A, Floege J. Hypoglycemia following intravenous insulin plus glucose for hyperkalemia in patients with impaired renal function. PLoS 1. 2017;12:e0172961.
-
Wheeler DT, Schafers SJ, Horwedel TA, Deal EN, Tobin GS. Weight-based insulin dosing for acute hyperkalemia results in less hypoglycemia: hyperkalemia treatment and hypoglycemia. J Hosp Med. 2016;11:355–7.
-
Harel Z, Kamel KS. Optimal dose and method of assistants of intravenous insulin in the management of emergency hyperkalemia: a systematic review. PLoS I. 2016;11:e0154963.
-
Capes SE, Chase D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of decease after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773–8.
-
King JT, Goulet JL, Perkal MF, Rosenthal RA. Glycemic control and infections in patients with diabetes undergoing noncardiac surgery. Ann Surg. 2011;253:158–65.
-
Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–67.
-
Allon M. Nebulized albuterol for acute hyperkalemia in patients on hemodialysis. Ann Intern Med. 1989;110:426.
-
Mandelberg A, Krupnik Z, Houri South, Smetana S, Gilad Eastward, Matas Z, et al. Salbutamol metered-dose inhaler with spacer for hyperkalemia: How fast? How prophylactic? Breast J. 1999;115:617–22.
-
Liou H-H, Chiang S-Due south, Wu S-C, Huang T-P, Campese VM, Smogorzewski Chiliad, et al. Hypokalemic furnishings of intravenous infusion or nebulization of salbutamol in patients with chronic renal failure: comparative study. Am J Kidney Dis. 1994;23:266–71.
-
Ellison DH, Felker GM. Diuretic treatment in heart failure. N Engl J Med. 2017;377:1964–75.
-
Reyes AJ. Effects of diuretics on renal excretory part. Eur Heart J. 1992;13:xv–21.
-
Reyes AJ. Renal excretory profiles of loop diuretics: consequences for therapeutic application. J Cardiovasc Pharmacol. 1993;22(Suppl 3):S11–23.
-
Chawla LS, Davison DL, Brasha-Mitchell E, Koyner JL, Arthur JM, Shaw AD, et al. Development and standardization of a furosemide stress test to predict the severity of astute kidney injury. Crit Care. 2013;17:R207.
-
Evans BM, Jones NC, Milne MD, Yellowlees H. Ion-substitution resins in the treatment of anuria. Lancet Lond Engl. 1953;265:791–5.
-
Harel Z, Harel S, Shah PS, Wald R, Perl J, Bong CM. Gastrointestinal adverse events with sodium polystyrene sulfonate (kayexalate) use: a systematic review. Am J Med. 2013;126:264.e9–24.
-
Bushinsky DA, Williams GH, Pitt B, Weir MR, Freeman MW, Garza D, et al. Patiromer induces rapid and sustained potassium lowering in patients with chronic kidney affliction and hyperkalemia. Kidney Int. 2015;88:1427–33.
-
Meaney CJ, Beccari MV, Yang Y, Zhao J. Systematic review and meta-analysis of patiromer and sodium zirconium cyclosilicate: a new armamentarium for the treatment of hyperkalemia. Pharmacother J Hum Pharmacol Drug Ther. 2017;37:401–11.
-
Bakris GL, Pitt B, Weir MR, Freeman MW, Mayo MR, Garza D, et al. Outcome of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA. 2015;314:151.
-
Stavros F, Yang A, Leon A, Nuttall K, Rasmussen HS. Label of structure and function of ZS-9, a Yard+ selective ion trap. PLoS ONE. 2014;9:e114686.
-
Kosiborod M, Peacock WF, Packham DK. Sodium zirconium cyclosilicate for urgent therapy of severe hyperkalemia. N Engl J Med. 2015;372:1577–8.
-
Lameire N, Van Biesen Due west, Vanholder R. Acute renal failure. Lancet. 2005;365:417–xxx.
-
Gaudry South, Hajage D, Schortgen F, Martin-Lefevre L, Pons B, Boulet E, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med. 2016;375:122–33.
-
Kovesdy CP, Regidor DL, Mehrotra R, Jing J, McAllister CJ, Greenland Due south, et al. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol. 2007;two:999–1007.
-
Pun PH, Lehrich RW, Honeycutt EF, Herzog CA, Middleton JP. Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int. 2011;79:218–27.
-
Morrison G, Michelson EL, Dark-brown S, Morganroth J. Mechanism and prevention of cardiac arrhythmias in chronic hemodialysis patients. Kidney Int. 1980;17:811–ix.
-
Ferrey A, You Every bit, Kovesdy CP, Nakata T, Veliz M, Nguyen DV, et al. Dialysate potassium and mortality in a prospective hemodialysis cohort. Am J Nephrol. 2018;47:415–23.
-
Roseman DA, Schechter-Perkins EM, Bhatia JS. Handling of life-threatening hyperkalemia with peritoneal dialysis in the ED. Am J Emerg Med. 2015;33:473.e3–5.
Authors' contributions
FD, ML: nerveless information, performed assay and interpretation of the data and drafted the manuscript. FP , KL , ZR , PR: performed analysis and estimation of the data and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The author thanks Pr. Bernard Canaud for his advice on the role of renal replacement therapy and editing of this part of the manuscript.
Competing interests
Dr. Dépret has nothing to disclose. Dr. Peacock reports grants and personal fees from Astra Zeneca, grants and personal fees from Relypsa, outside the submitted piece of work. Dr. Liu reports grants from NIH: National Middle, Lung and Blood Plant, grants from NIH: National Institute of Diabetes and Digestive and Kidney Disease, personal fees from Achaogen, personal fees from Durect, personal fees from Z South Pharma, personal fees from Theravance, personal fees from Quark, personal fees from Potrero Med, other from Amgen, grants from American Social club of Nephrology, grants from National Kidney Foundation, grants from National Policy Forum on Disquisitional Intendance and Acute Renal Failure, personal fees from Baxter, exterior the submitted work. Dr. Rafique reports personal fees and other from AstraZeneca, grants and personal fees from Vifor, exterior the submitted work. Dr. Rossignol reports personal fees from French National Enquiry Bureau Fighting Heart Failure (ANR-15-RHU-0004), personal fees from French PIA project «Lorraine Université d'Excellence» GEENAGE (ANR-15-IDEX-04-LUE) programs, outside the submitted work. Dr. Legrand reports grants from French ministry of health, grants and nonfinancial support from Sphingotec, personal fees from Fresenius, personal fees from Baxter-Hospal, and personal fees from Novartis, outside the submitted work.
Availability of data and materials
Not applicable.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Non applicable.
Funding
Non applicable.
Publisher's Annotation
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Affiliations
Corresponding author
Boosted file
Additional file 1: Figure S1.
Gastrointestinal absorption site of ZS-nine, SPS and patiromer. The majority of potassium is in the distal gastrointestinal (GI) tract (due east.g., the colon). Both sodium polystyrene sulfonate (SPS) and patiromer are concentration dependent binding (with patiromer being improve than SPS). Since there is not relatively much potassium in the early on office of the GI tract, SPS and patiromer take less of an result because at that place is less for them to bind. Furthermore divalent cation (Ca2+ and Mg2+) are inadvertently pick up as well. On the contrary, sodium zirconium cyclosilicate (ZS9), which is much more attracted to potassium and more specific than SPS or patiromer (binding coefficient much college), that it can bind potassium in low concentration environments with less competition with divalent cation, so it starts binding earlier in the GI tract.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/iv.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(southward) and the source, provide a link to the Artistic Commons license, and indicate if changes were fabricated.
Reprints and Permissions
About this commodity
Cite this article
Dépret, F., Peacock, West.F., Liu, Grand.D. et al. Management of hyperkalemia in the acutely sick patient. Ann. Intensive Care 9, 32 (2019). https://doi.org/ten.1186/s13613-019-0509-eight
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/x.1186/s13613-019-0509-8
Keywords
- Hyperkalemia
- Intensive care
- Emergency
- Renal replacement therapy
- Acute kidney injury
Source: https://annalsofintensivecare.springeropen.com/articles/10.1186/s13613-019-0509-8
Post a Comment for "What Laboratory Reports Will the Nurse Review Before Administering Albumin to a Patient?"